TRanscripts to Identify Meningitis - TRIM study
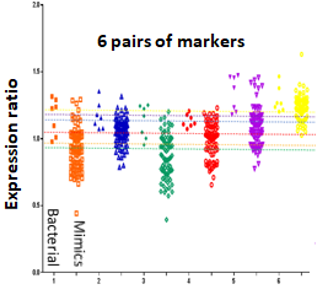
Bacterial meningitis remains a significant cause of morbidity and mortality in the UK and worldwide, with considerable costs. The key step in early patient management is to distinguish bacterial meningitis, which needs immediate antibiotic treatment, from the many mimics, such as viral meningitis, which do not. The essential test for distinguishing the two is the lumbar puncture. Currently, cerebrospinal fluid (CSF) is collected via lumbar puncture (LP) and bacterial culture undertaken.
However, LPs can be delayed and CSF tests inconclusive (McGill, Griffiths et al. Lancet Infectious Diseases 2018 ). Many patients are therefore treated with unnecessary antibiotics until a diagnosis of bacterial meningitis is excluded, contributing to extended stay in hospital and potentially increasing the risk of complications such as antimicrobial resistance.
Our novel, rapid blood test measuring TRanscripts to Identify bacterial Meningitis (TRIM test) offers to accurately distinguish bacterial meningitis from clinical mimics using the body’s host response pattern in blood. Test use will speed-up diagnosis and in-turn improve management and reduce unnecessary antimicrobial use. European and USA patent has been obtained.
Our prototype host-transcript assay was developed by the team. In 2019 we commenced our Clinical Diagnostic study funded by MRC DPFS and Fast Track Diagnostics (FTD), Luxembourg. Following development of a large hospital network across the UK, Denmark and Netherlands, clinical sample (blood / CSF) collection from patients (n>700) with suspected meningitis has been achieved.

Photo: Meeting of the European Leads of the TRIM network in Aarlborg, Denmark.
The assay was modified (e.g. translated into a lyophilised format) for commercial use in collaboration with FTD. FTD was later bought out by Siemens, and their research priorities changed. In 2022, we established new collaborations with UK industrial partners – MedtechtoMarket (M2M) and Dam Health. We have successfully secured further funding from MRC-DPFS, with a costed project extension until October 2025.
The primary objective of the TRIM test study is to validate the accuracy of the prototype TRIM test in UK and European adults and translate the TRIM assay into a viable commercial diagnostic test for clinical use.
Currently. the TRIM assay is continuing to undergo commercial translation. M2M are translating the TRIM assay for use on different thermocycler platforms. We are developing external and internal controls to be integrated into the assay. We are also developing interpretative software for the assay with another commercial partner, Ugentec, Belgium.
We plan a further clinical validation of the prototype assay in 2023. Once we have completed commercial development of the TRIM test in adults, we will examine its accuracy in children and lower-middle income populations. This is planned for 2024-2025.
The TRIM study is also collecting data on the length of stay, antibiotic prescribing and patient outcomes among suspected adult meningitis cases admitted to UK hospitals. Initially, we plan to estimate the potential health and economic benefits for a bacterial meningitis diagnosis to be confirmed or ruled out among UK suspected meningitis patients recruited via the study (based on number of patients and number of in-patient days to diagnosis). After this, we plan to examine the health and economic benefit of the TRIM test in clinical use compared to routine diagnostics at NHS hospitals as part of a NIHR HTA study.